Gucy2c Car T
These observations highlight the potential for therapeutic translation of GUCY2C-directed CAR-T cells to treat metastatic tumors without collateral autoimmunity in patients with metastatic colorectal cancer. Thus we have identified a human GUCY2C-specific CAR-T cell therapy approach that may be developed for the treatment of GUCY2C-expressing metastatic colorectal cancer.

Human Gucy2c Targeted Chimeric Antigen Receptor Car Expressing T Cells Eliminate Colorectal Cancer Metastases Cancer Immunology Research
Importantly the therapeutic effects of GUCY2C-CAR T cells were not associated with antigen-dependent clinical toxicity including diarrhea rectal bleeding or rectal prolapse or gross pathology including hemorrhage or ulceration in intestine.
Gucy2c car t. Ad Online Chat Support. All the top makes. Repair Manuals Service Manuals Workshop Manuals ECP Diagnostics.
GUCY2C murine CAR-T cells recognized and killed human colorectal cancer cells endogenously expressing GUCY2C providing durable survival in a human xenograft model in immunodeficient mice. GUCY2C-negative CRC cells were not killed by either. GUCY2C CAR-T cells provided long-term protection against lung metastases of murine colorectal cancer cells engineered to express human GUCY2C in a syngeneic mouse model.
They found that GUCY2C CAR-T cells reduced the number of metastatic tumors in mices lungs and the treated-mice showed reduced morbidity and improved survival without inducing autoimmunity. GUCY2C murine CAR-T cells recognized and killed human colorectal cancer cells endogenously expressing GUCY2C providing durable survival in a human. They designed a CAR-T cell that recognized the GUCY2C tag and showed the approach was effective in killing tumors and preventing metastatic growth in mice.
Human GUCY2C-targeted murine CAR-T cells promoted antigen-dependent T-cell activation quantified by activation marker upregulation cytokine production and killing of GUCY2C-expressing but not GUCY2C-deficient cancer cells in vitro GUCY2C CAR-T cells provided long-term protection against lung metastases of murine colorectal cancer cells engineered to express human GUCY2C in a syngeneic. Murine CAR-T cell generation. Ad Lentiviral Vetors and DNA-encoded mAbs for Car-t Therapy Targeting Gucy2C.
Murine CAR components were used to produce a third-generation codon-optimized retroviral CAR construct as previously described A codon-optimized scFv sequence derived from the 5F9 human GUCY2C-specific antibody Supplementary Fig. Ad Lentiviral Vetors and DNA-encoded mAbs for Car-t Therapy Targeting Gucy2C. Instant workshop manual download.
Results GUCY2C-directed CAR-T cells specifically lysed the GUCY2C-expressing metastatic CRC cell line T84 while the control CAR did not. Human GUCY2C-targeted murine CAR-T cells promoted antigen-dependent T-cell activation quantified by activation marker upregulation cytokine production and killing of GUCY2C-expressing but not GUCY2C-deficient cancer cells in vitro GUCY2C CAR-T cells provided long-term protection against lung metastases of murine colorectal cancer cells engineered to express human GUCY2C in a. In addition to cell killing GUCY2C-directed CAR-T cells of both the CD8 and CD4 co-receptor lineage produced the inflammatory cytokines IFN-γ and TNFα in response to GUCY2C antigen.
High Quality CarTcr Products for Immunotherapy Research. The study Human GUCY2C-targeted chimeric antigen receptor CAR-expressing T cells eliminate colorectal cancer metastases was published in the journal Cancer Immunology Research. GUCY2C CAR-T cells oppose metastatic colorectal cancer Mice received CT26GUCY2C cells by tail vein to induce lung metastases31 followed 3 d later by 5 Gy total body irradiation TBI and 1 107 T cells32 A non-myeloablative dose of 5 Gy TBI was administered prior to T cell transfer to enhance the.
Moreover treatment with GUCY2C CAR-T cells did not show toxicity and accumulation in the mice intestine. GUCY2C-directed T cell efficacy reflected CAR affinity and surface expression and was achieved without immune-mediated damage to normal tissues in syngeneic mice. Ad Online Chat Support.
Instant workshop manual download. Snook and colleagues then showed that the CAR T-cell therapy using the GUCY2C tumor antigen successfully treated mice implanted with human colorectal cancer tumors. S2 was cloned into a CAR construct containing murine sequences of the BiP signal peptide CD8α hinge region.
Number of participants with CAR-T treatment-related adverse events as assessed by CTCAE v403 Time Frame. All the top makes. 24 months The investigator is responsible for ensuring that all adverse events observed by the investigator or reported by the subject during the 3-month period from enrollment ie.
While the researchers now focus on their adenovirus-based vaccine trial they are also planning a GUCY2C CAR-T. The concept of moving CAR-T cell therapy to colorectal cancer is a major breakthrough and could address a major unmet clinical need. High Quality CarTcr Products for Immunotherapy Research.
Initiation of leukocyte separation to 3 months after targeted car-t infusion are monitored and reported. The T cells are genetically modified through transduction with a lentiviral vector expressing scFv of anti-GUCY2C antibody linked to 41BB and CD3ζ signaling domains. Repair Manuals Service Manuals Workshop Manuals ECP Diagnostics.
The vector of anti-GUCY2C chimeric antigen receptor CAR is constructed for the engineering of T cells to target Human GUCY2C. Moreover these CAR T cells extended median and overall survival in therapeutic mouse models of GUCY2C-expressing colorectal cancer metastatic to lung.

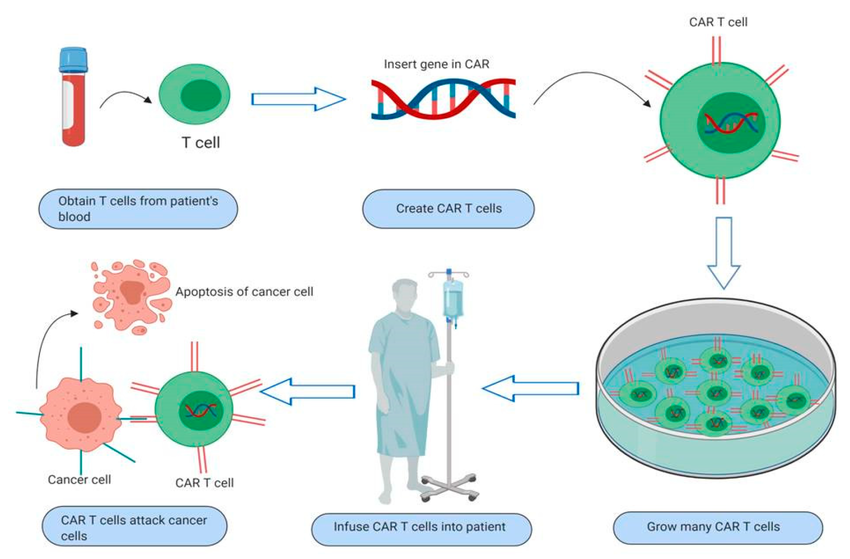
Overview Of Chimeric Antigen Receptor Car T Cell Therapy Process Of Download Scientific Diagram

Human Gucy2c Targeted Chimeric Antigen Receptor Car Expressing T Cells Eliminate Colorectal Cancer Metastases Cancer Immunology Research

Overview Of Chimeric Antigen Receptor Car T Cell Therapy Process Of Download Scientific Diagram
Https Www Tandfonline Com Doi Pdf 10 1080 2162402x 2016 1227897 Needaccess True